Is your idea the next game-changer?
Together we create the next generation of medical technology


Medical device consultants
We support medical device companies through innovation, market clearance and growth. CE-marking of medical devices is in our DNA. We have supported hundreds of Swedish medical device innovators since starting Medos in 2006. We offer a broad service and product portfolio to support Swedish healthcare innovators.
News!
To further enhance our services in the Medicon Valley, we have now opened up an office in Helsingborg

Let us develop your software
We can onboard your product idea and start a development project with full medical device management in a cost-effective way, allowing you to grow into your role as a manufacturer at your own pace. If you need development resources, we are by your side.
-
Trusted Development Partner
-
Proven Project Model
-
Agile Methodology
-
All the way to market
Now we need to strengthen our team!
› App and Web Developer with a sense for UX
› Consultant in Quality and Regulatory Affairs for Medical Technology
Expert medical device consultant services for regulatory success
Looking for a reliable medical device consultant to guide you through regulatory pathways and product development? At Medos, we specialize in helping medical device companies navigate the complex landscape of global compliance. As an experienced medical device consultant, we offer expert support across all stages — from idea to market.
Our services cover EU MDR, IVDR, ISO 13485, risk management, clinical evaluations, and quality management systems. Whether you're a startup or an established manufacturer, our team of medical device consultants tailors solutions to your specific needs, ensuring speed, compliance, and efficiency.
Working with a medical device consultant from Medos means gaining access to deep industry knowledge, hands-on regulatory experience, and a results-driven mindset. We don’t just advise — we work alongside your team to get things done.
Choose Medos as your trusted medical device consultant partner and benefit from a flexible, scalable, and cost-effective approach. We understand the demands of the medtech industry and deliver solutions that help you succeed.
Contact Medos today and see how a skilled medical device consultant can accelerate your path to market and compliance.
Webinars to support your ISO certification
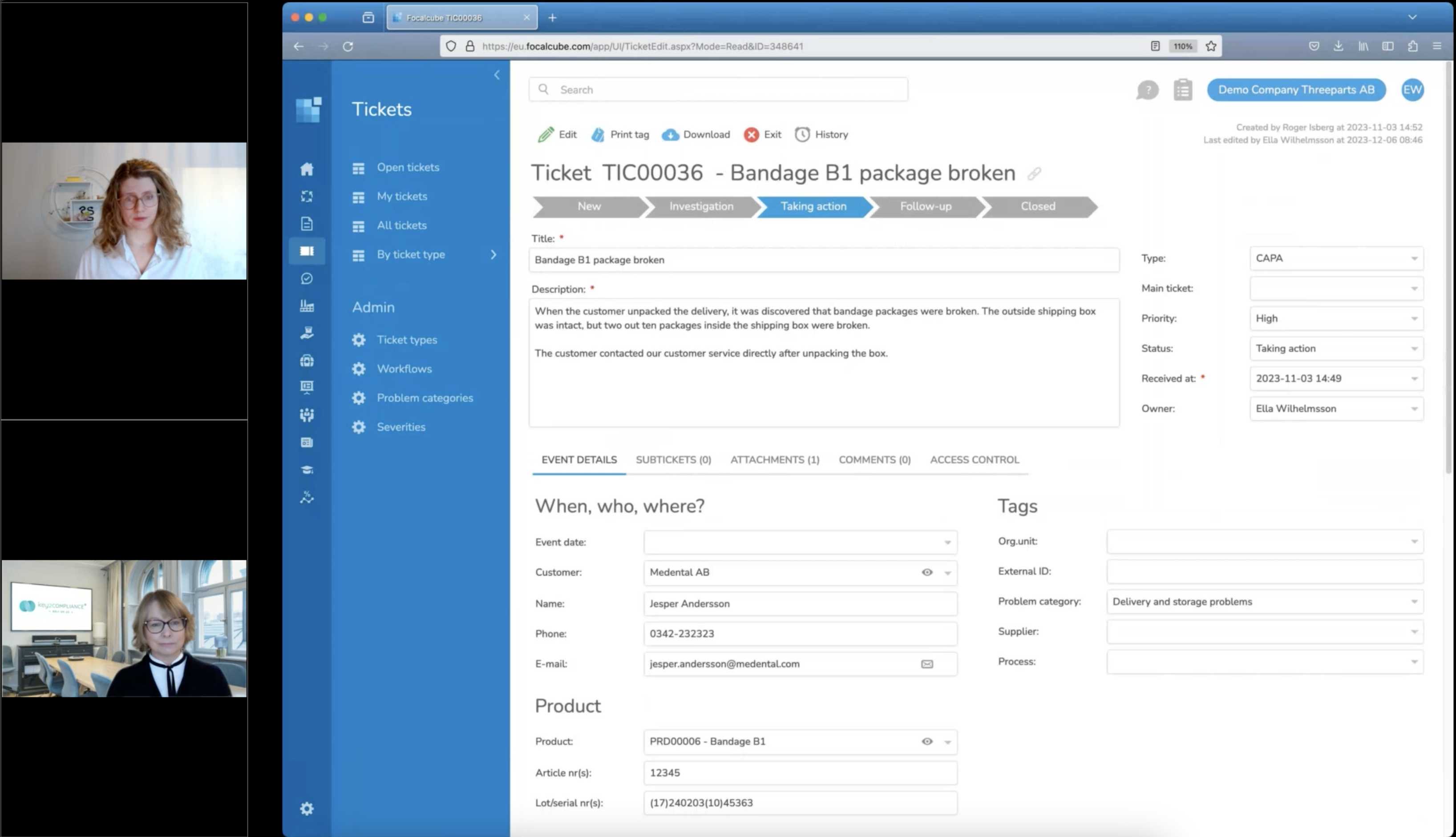
CAPA
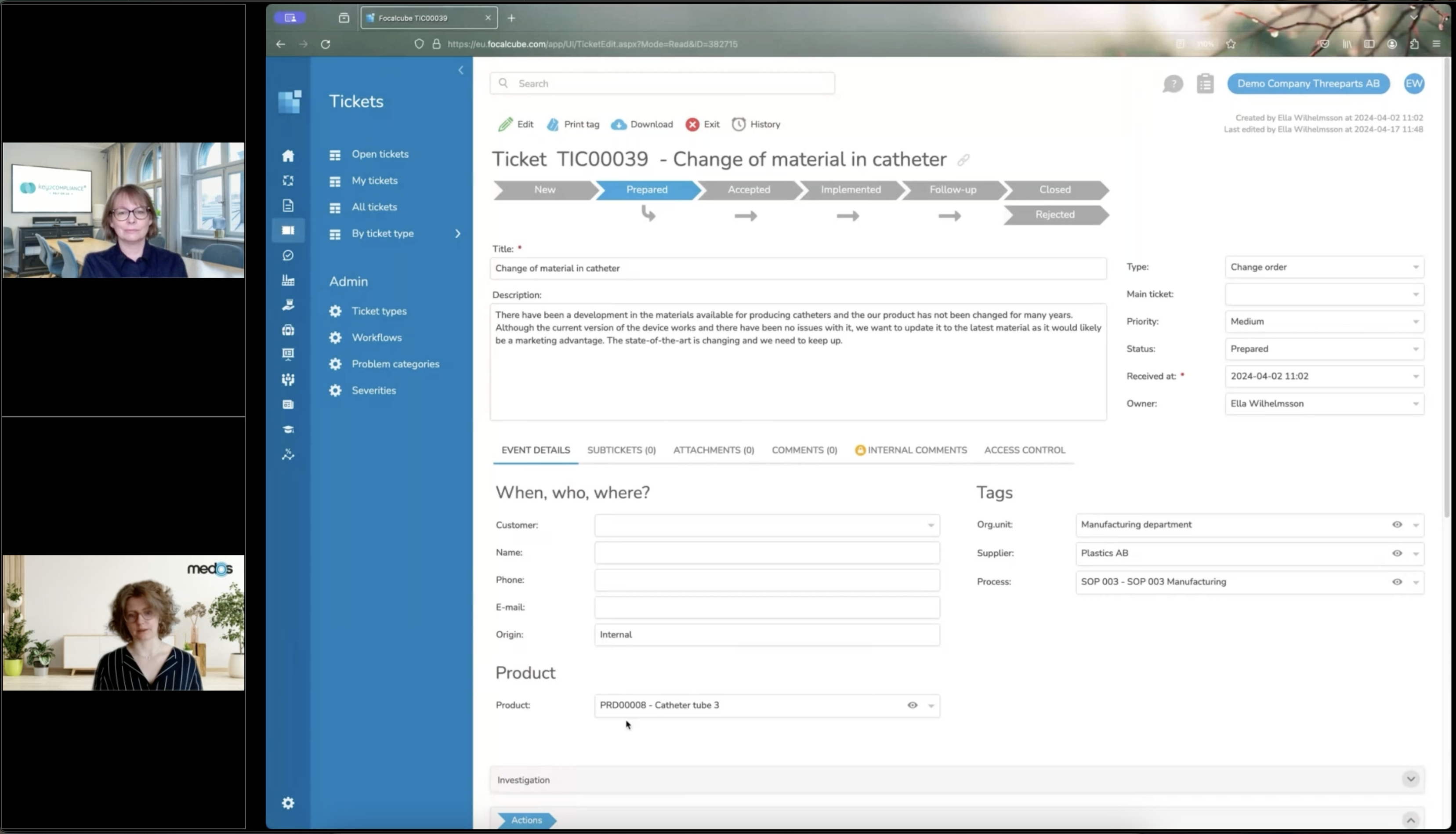
Change management
Five Success Factors for Startups in Medical Device Software
Do you have an amazing product idea in the works?
We have worked with many innovation companies over the years. Here, we share some of what we've learned. Feel free to check out the article and reach out if you'd like to discuss how we could assist you!